One-Stop Innovation Shop Hydrix Poised for Growth
Plenty of companies assert that “innovation” is their guiding principle, but Melbourne-based Hydrix Limited takes it more literally than most.
Four years ago, when it arrived on the ASX through a merger with the listed Panorama Synergy, Hydrix was a product engineering consultancy that designed and developed prototypes for clients from around the world, from small Australian companies to global household names such as Siemens, HP, GE and Philips.
The company has expanded that product innovation capability to encompass three major growth platforms, namely:
• Hydrix Services – which aims to design and engineer client products capable of transforming industries;
• Hydrix Medical – which works to bring innovative medical technologies to market; and
• Hydrix Ventures – established to invest in the company’s high-potential clients, to generate equity returns.
While these are complementary businesses, the link is Hydrix’s core design and engineering capabilities, which is applicable in a range of areas, including medical technology, mining, utilities, aerospace and defence.
However, Hydrix is not just an engineering business – it has added to its range of services non-engineering capabilities in the areas of project management, regulatory expertise, clinical, reimbursement, market insights and commercialisation, so as to offer what it describes as “a unique one-stop-shop with the ability to bring ideas from concept through to market.”
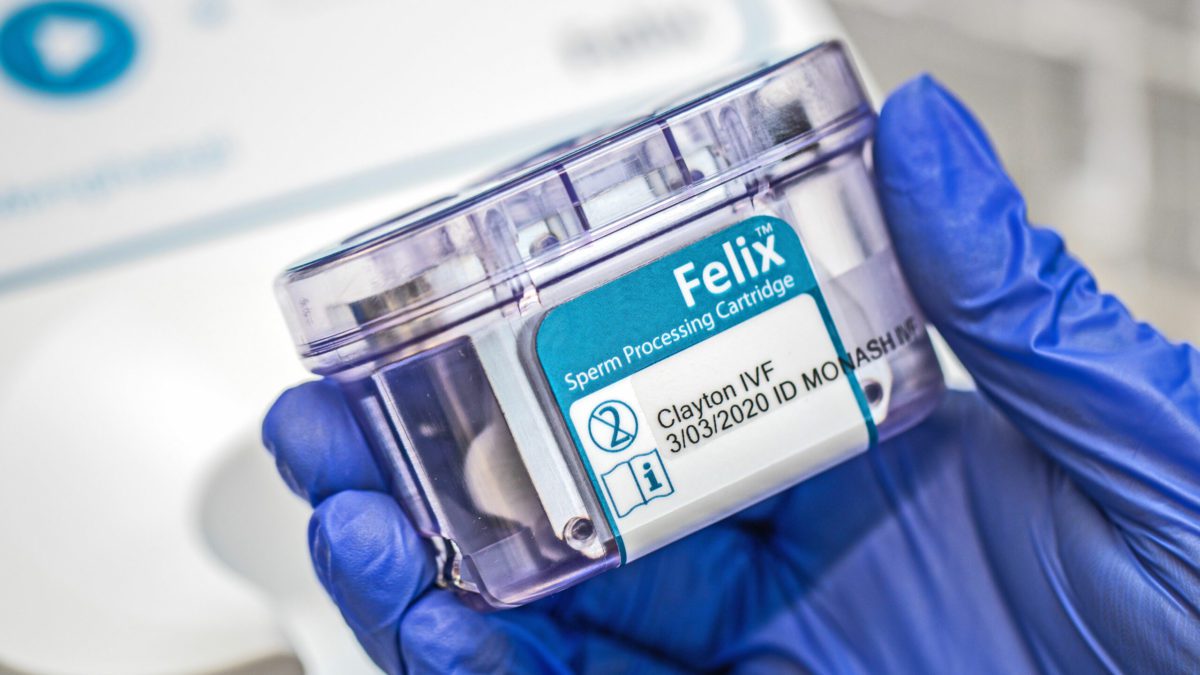
Medical technology is firmly in Hydrix’s DNA. For example, the company worked on some of the major medical device success stories of the ASX, such as Nanosonics’ (ASX:NAN) Trophon sterilizers, Memphasys’ (ASX:MEM) Felix sperm separation device, LBT Innovation’s (ASX:LBT) culture plate automation devices and Micro-X’s (ASX:MX1) portable x-ray device. Last year, Hydrix and Memphasys both received 2020 Good Design Awards for the design of an instrument and cartridge system used to improve outcomes in IVF.
The FELIX automates the extraction of the highest-quality sperm from a semen sample, through a quick, consistent and controlled process, that is intended to increase the likelihood of couples successfully conceiving and giving birth through IVF procedures. Hydrix’s role in developing this device was to evolve an initial technology concept into a commercially viable instrument and cartridge that was compact, intuitive and safe to use, reliable, and laboratory-friendly.
While Hydrix retains a small (1%) investment in Memphasys, the strategy now is to make deeper investments in high-potential medical innovation.
A good example is the company’s relationship with New Jersey-based Angel Medical Systems, which has developed the AngelMed Guardian medical device, which is billed as the first implantable, patient-alerting system designed to warn patients to seek medical attention for Acute Coronary Syndrome (ACS), including heart attacks.
In July 2019, Hydrix acquired the exclusive Asia-Pacific rights to distribute the Guardian device in eight countries across the Asia-Pacific region, including Australia, Singapore, and Japan, for an initial period of seven years. Hydrix Services provides input into the design of the Guardian system; and Hydrix Ventures has invested in AngelMed, to generate potential equity capital gains from AngelMed’s future global success. At present Hydrix owns 4.6% of AngelMed.
The AngelMed device is designed to detect abnormal patterns in the electronic signal of each patient’s heart. It then activates a warning system, effectively acting as a preventative measure for symptoms of ACS, such as silent heart attack
In August 2020, Hydrix announced that the device had been successfully implanted in four patients in Singapore. That was followed in October by the first successful implant of the device in a US patient. as part of a continued access study with the US Food & Drug Administration. Six devices have now been implanted, five in Singapore and one in the US.
The implanting surgeon, Kelly Tucker, described the AngelMed device as a “game-changing” technology for people with heart problems. “This is the first-ever surveillance tool for patients with a prior coronary event and has the potential to save countless lives and bring peace of mind to thousands of families,” Tucker said.
AngelMed can be considered the lead-product in what Hydrix plans to be a portfolio of medical devices for detection and treatment of cardiovascular and neurovascular diseases, collectively the biggest causes of death globally. In October last year Hydrix signed a deal to distribute) in Australia and New Zealand) California-based company’s Phyzhon Health’s disposable fibre-optic pressure sensor “guidewire,” which is used for interventional cardiology procedures: the novel product improves the stent selection and insertion by interventional cardiologists for patients with coronary artery disease, improving patient and healthcare outcomes. Hydrix’s exclusive four-year-plus distribution rights deal has a potential annual market demand of up to 125,000 units.
In December, Hydrix announced a first-in-human (FIH) trial of the guidewire – full name, PHYRARI FFR (Fractional Flow Reserve)-WIRE – at a Melbourne-based teaching hospital under the Australian Therapeutic Goods Administration’ (TGA) Clinical Trial Notification (CTN) scheme. Subject to the timing of US Food & Drug Administration (FDA) and TGA approval, Hydrix expects to begin commercial distribution of the guidewire in Australia by the end of 2021.
Hydrix is also working with Cyban Pty. Ltd., an Australian technology company focused on developing a novel, non-invasive continuous tissue oxygen monitoring device for continuous measurement of brain tissue oxygen levels in patients who have suffered a brain trauma injury. Hydrix owns 7.5% of Cyban.
Another client is Melbourne-based Gyder Surgical, which is developing a novel medical device to help surgeons achieve more reliable and consistent hip replacement surgery outcomes. Gyder Surgical’s device helps surgeons to ensure that the artificial hip socket is positioned accurately in the pelvis. Currently, many surgeons perform this ‘navigation’ freehand, which leads to an average of only 50% alignments being correct. The consequence of this is an estimated and avoidable annual cost of up to US$2 billion ($2.6 billion) in the US alone for revised hip surgeries.
Hydrix owns up to 15% of Gyder Surgical; in return, Gyder says it is “taking advantage of having R&D, regulatory and quality systems skills on-call, and under the one roof.”
“We describe ourselves as a ‘powerful product innovation company’,” says Hydrix executive chairman Gavin Coote. “We say that we’re a one-stop-shop that can help our clients take ideas from concept through to market, because we’ve got all the skill-sets, from design to regulatory, to help clients transform an idea into a commercial product.
“We’re not a contract manufacturer – part of what we do is find the most appropriate contract manufacturing partner for a client. It’s a pretty unique business, and sometimes that can work to your detriment on the stock exchange, because the market likes to compare you with something. We’ve brought all the parts of the innovation and commercialisation ecosystem together. Quite simply, Hydrix is just a collection of highly skilled and talented people who excel at solving lots of complex problems for clients,” Coote says.
While not yet profitable, Hydrix was in a strong cash position at half-year, having raised $13 million in a share placement and extinguishing $3.5 million of secured debt, leaving the company with $9.2 million of cash on hand at 31 December 2020. In its half-year report, Hydrix said it anticipated high potential Hydrix Medical cardiac product revenue and margin growth beginning FY22; profitable growth in the core design and engineering services business beginning 2H FY21; and capital returns from the high-potential Ventures investee companies, “all of which have significant product advancement and capital raising milestones in the year ahead.”
From 26 cents at the start of the year – and 28 cents in the October 2020 placement – HYD has retraced to 21 cents, which capitalises it at $34 million. For those that are truly interested in innovation, Hydrix might well reward further research.